Anyone who has traveled to London and taken the “tube” has heard the monotoned robotic recording upon the arrival of every train – Mind the Gap…mind the gap…mind the gap. In other words, watch the gap between the train and platform when boarding the train. We can make a similar correlation with good supply chain management and documentation. Let’s call it “Mind the Doc”; in other words, pay close attention to the documentation.
One of the many factors of good supply chain management could be right in front of you. In fact, it might even be you! Are you involved with product development, sourcing, production, logistics, or the information systems that manage these activities? Have there ever been changes in any of these areas? If the answer is “Yes” then we encourage you to continue reading.
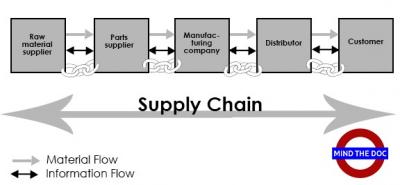
Supply chain management has been defined as the collective activities of the supply chain to maximize value and achieve competitive edge. These activities determine how effectively and efficiently the products reach their end users. Supply chains involve multiple organizations that are all linked together with the physical product and informational flow to reach a common goal – the on-time delivery of quality products to the customer.
In the competitive world of printed circuit board assembly (PCBA), it’s critical to deliver products in a timely manner and without defects. Similar to raw material suppliers, printed circuit board assemblers are in the early stages of the supply chain – flowing materials and products “upstream” to manufacturers. To incur late deliveries or non-conformities this early in the supply chain will interrupt production, waste time, incur unnecessary scrap, and ultimately strain business relations.
The good news is that we can all play a role in improving our supply chains. Let’s call it “MIND THE DOC”. When changes to drawings, Gerber files, schematics, bill of materials, etc. occur, update all documents immediately. Otherwise, it is likely that future problems are imminent. It could be that a component becomes obsolete or has a long delivery time and a replacement was found and approved – but failed to be documented. Maybe the change was a new design or firmware for board performance or enhanced manufacturability – and for a plethora of reasons did not get documented. These changes, often made in the best interest of the customer, are one of the leading causes of non-conformity. Sound familiar? Most of us would admit we’ve fallen victim to a similar scenario.
As a manufacturer, we know how fast-paced manufacturing can be. There are a lot of moving parts, looming deadlines, running short on time, and it’s easy for something to fall through the cracks – particularly documentation updates. Believe us when we say, we know! We get it. But seriously, maintaining accurate documentation is a common area that we all can play an active role to improve the supply of materials and products. When changes occur, regardless of how small, let’s all remember to “MIND THE DOC” and update changes (i.e. revision level) immediately. As our wise Uncle Ben once said, “An ounce of prevention is worth a pound of cure”.